
How Software Quality Engineering and Design Quality Are Similar

REFLECTION: FOR STUDENTS: If your education looks as if it may lead to a point where you have interactions that involve a QMS, be sure to pursue at least a basic understanding of software, as that understanding will be gold in all future industry employment.
FOR ACADEMICS: AI and ML are coming, but it will be a while before any doctors or engineers are actively replaced, but it would be wise to teach your students how to think for yourself, not just rely upon the computer. Teach students to be the person who writes the program or qualifies the program, not the person who is told by the computer what to do.
FOR PROFESSIONALS/PRACTITIONERS: Learn all you can about the software world if you have not yet. If you are purely a Software Quality Engineer, get as much exposure to other quality systems to help open your mind up to different problem-solving pathways. Always be a lifelong learner.
Definition of Quality
There is no single definition of quality that has ever or likely ever will be agreed upon even in a single company, much less an entire industry or across industries, but the ISO/IEC/IEEE Systems and Software Engineering-Vocabulary (ISO/IEC/IEEE, 2010)
provides an excellent layout of every aspect of defining quality.
- The degree to which a system, component, or process meets specified requirements
- The ability of a product, service, system, component, or process to meet customer or user needs
- The totality of characteristics of an entity that bears on its ability to satisfy stated and implied needs
- Conformity to user expectations, conformity to user requirements, customer satisfaction, reliability, and level of defects present
- The degree to which a set of inherent characteristics fulfills requirements
Each organization must decide what quality is, however, only a world-class organization will always be using the feedback from all stakeholders to evolve the organization’s definition of quality so that stakeholders are satisfied at every level.
Changes Are Coming
I have written a couple of posts about how Quality 4.0 and Industry 4.0 are here. However, based upon the fact that the Quality industry is in many areas still building the foundations of a Quality culture that will support Quality 4.0, there are likely to be many quality professionals who fall into one of three major groups.
Some may be in a state of denial or unawareness of the exponential growth level of the emerging technology and believe things will stay the same as they have for all these years. The next group is made up of those who are fully aware of the changes that are coming. The members of this group and are getting ready for the inevitable change. The last, and most likely smallest group (though I have no formal data, shame on me), would be those who know what is coming but actively do not want the change to occur.
The reason I believe that would be the smallest group would be the fact that the vast majority of quality professionals are problem solvers, change agents, and not comfortable with things just staying as they are. The old “If it isn’t broke, don’t fix it” axiom is the antithesis of the Quality mindset.
Software Quality Engineering
In the past few years, I have witnessed the medical device industry evolve rapidly. A few years ago, I saw DHFs (Design History File), DMRs (Device Master Record), and DHRs (Device History Record), all kept on paper in files in a locked room and carefully controlled. Quickly the entire paradigm shifted to Cloud storage of these documents with ultra-tight security, in addition to management of routing of document approvals moving from physical signatures to electronic signatures. I also witnessed software become officially regulated as medical devices (SaMD).
Software Quality Engineering is- The study and systematic application of scientific technology, economic, social, and practical knowledge, and empirically proven methods, to the analysis and continuous improvement of all stages of the software life cycle to maximize the quality of software processes and practices, and the products they produce. Generally, increasing the quality of software means reducing the number of defects in the software and in the processes used to develop/maintain that software (WestFall, 2016). Software development usually uses an Agile project management approach. Agile project management seems to draw heavily from the Lean philosophy, which focuses on creating better customer value while minimizing waste. Both philosophies emphasize fast deliverables. The emphasis of Lean is on reducing waste and unnecessary steps; however, Agile emphasizes breaking large tasks into small ones and delivering in short sprints. Both Lean and Agile use some sort of an action loop. In Lean, this is the build-measure-learn cycle, while Agile’s scrum methodology uses an iterative sprint approach (L. Allison Jones-Farmer, 2016).

The iterative steps used as part of the Agile Scrum methodology help prevent excessive costs. When a defect is not caught early in the development of software, the defect can impact many more aspects of the software. The later in the life cycle the defect is detected, the more effort (and therefore expense) it will take to isolate and correct defects. A single defect in the requirement phase may have a “butterfly effect” and cause exponential levels of cost as the defect goes undetected.
At the end of each iteration, the goal would be to have a working example demonstratable for stakeholder review and feedback so that any issues with correctness or quality can be addressed in the next iteration (WestFall, 2016).
Traditional Design Quality
Design Quality from the manufacturing perspective aligns closer to Crosby’s viewpoint of the need for a well-defined specification against which to measure quality. One of the things about the fast-changing Quality world is that software is less about the reproducibility to spec and more about innovation. As the manufacturing world has found itself thrown into a massive acceleration in the last few decades, traditional design perspectives have been re-evaluated. Engineering tools like TRIZ have been employed to help spur innovation in design and problem-solving. Design For Six Sigma (DFSS) shifted product design away from just doing it right the first time toward robustly employing QFD to obtain the voice of the customer. The best DFSS process for a new design (in my opinion) would be the IDOV method- Identity (obtain VOC & CTQs), Design, Optimize, Validate. At the Validate stage, a prototype is tested and validated, with risks thoroughly analyzed (and as required, sent back to an earlier stage if validation is not accepted) (Kubiak, 2017). I have seen more and more manufacturing operations learning to use agile methods for design, frequently combining Agile with Lean, Six Sigma, or Lean Six Sigma depending upon the culture and knowledge base. It is clear that as software, apps, and AI become integral parts of product and production, the pace at which innovation must occur to meet customer requirements will continue to grow, perhaps eventually leading to a permanent fusion of Agile, Lean, and Six Sigma.
Conclusion
There are many other methodologies out there, like the waterfall mythology, which has lost a bit of popularity over the past few years to Agile methods, as well as CMMI, which I have heard conflicting reports on how compatible CMMI is with Agile. I would appreciate input from those in the software industry who can tell me firsthand how well CMMI and Agile work together. Software DevOps is effectively designing a product for stakeholders from ideation to operational release and monitoring/maintenance. Designing a physical item to sell on the market based upon the VOC using a QFD may be slower and use a more thorough investigation of the requirements and desired outputs (since you are addressing something tangible rather than an idea for a program). An idea that can provide value (software) is much harder to define in a neat specification box. Still, just like a DOE, you keep adjusting your settings and continue trying until you determine what factors are critical. Then you optimize the settings for your critical factors to obtain the best outcome based upon cost, functionality, and VOC. If you are worried about the coming changes, do not be worried, embrace the change, and be part of the change. Yes, in 20 years, we will all be behind a computer console writing code (if AI has not replaced al the Quality Engineers), but as long as you are looking forward, you will be moving in the right direction.


Bibliography
ISO/IEC/IEEE. (2010). ISO/IEC/IEEE 24765 Systems and Software Engineering. Geneva, Switzerland: ISO New York, NY.
Kubiak, T. a. (2017). The Certified Six Sigma Black Belt Handbook Third Edition. Milwaukee: ASQ Quality Press.
L. Allison Jones-Farmer, P. T. (2016, 10). asqstatdiv.org. Retrieved from ASQ: http://asq.org/statistics/2016/10/agile-teams-a-look-at-agile-project-management-methods.pdf
WestFall, L. (2016). The Certified Software Quality Engineer Handbook 2nd Edition. Milwaukee, WI: ASQ Quality Press.
The Technology for Industry 4.0 is Here, but Quality 4.0 is at 3.0 Going on 4.0 (With Still More Growing To Do)!

REFLECTION: FOR STUDENTS: Are the current Management frameworks you have been taught sufficient for Industry/Quality 4.0, or will you have to shift your perspective to be viable?
FOR ACADEMICS: Are you teaching two years behind (as is common with textbooks) or are you also including the coming impact of Quality 4.0 and disseminating that required knowledge to your students?
FOR PROFESSIONALS/PRACTITIONERS: Always focus maintaining and improving on the current state, but plan for the achievement of the future state or the future state will never be realized.
Industry 4.0
For many years, the quality culture has been the lighthouse that lit the way down the path, guiding industry toward the reduction of waste and higher levels of customer satisfaction and retention, but things have been evolving. Industry has gone through many changes, and those changes always have required Quality to adjust.
Industry revolution 1: machine manufacturing, steam power, and the migration to city living for people who had previously been agriculturalists. (Quality moved from guilds into the role of inspection)
Industry revolution 2: The production line and mass manufacturing drastically reduced the cost of consumer and industrial products. (Quality developed more efficient quality management tools)
Industry Revolution 3: Barely a revolution, but significant. Electronics and control systems have gradually penetrated manufacturing, allowing greater flexibility and more sophisticated products at a significantly lower cost until ERP and PLM have become the standard. (Quality began to utilize data to analyze costs/customer complaints and reduce enterprise costs due to quality failures)
Each industrial revolution brought about new patterns of quality management. As Deming emphasizes, if the industrial culture is not Quality-oriented, it will ultimately displease the customer. Still, right now, the fourth industrial revolution is upon us, and Quality, though fully aware of the future state goals, is still working on perfecting the foundations of Quality 3.0. At the same time, Industry 4.0 is off and running.
Industry 4.0 consists of Many critical technology changes and advancements that have enabled technology to become more predictive and communicative, including advances in data, analytics, connectivity, scalability, and collaboration. The digital impact of the third revolution is magnified dramatically. It evolves industry into a phase where AI helps solve problems proactively rather than picking through the past failures to figure out what went wrong. This phase will touch every aspect of industry, connecting people, machines, and data in new ways, and provides access to technologies that were previously only accessible to a skilled few and heralds transformative capabilities such as those in material science and 3D Printing.

Adapted from Quality 4.0 Impact and Strategy Handook
What is Quality 4.0?
These are the primary axes of Quality 4.0 I want to discuss:
- Data
- Analytics
- Connectivity
- Collaboration
- Scalability
- Management Systems
- Compliance
- Culture
The effect of the implementation of these technologies is essential to quality because they allow for the transformation of culture, leadership, collaboration, and compliance. Quality 4.0 is genuinely not about technology, but the users of that technology, and the processes they use to maximize value. Quality 4.0 doubtlessly includes the digitization of quality management. It is more important to consider the impact of that digitization on quality technology, processes, and people. Quality 4.0 should not be sold as a buzzword system to replace traditional quality methods, but rather as a framework designed to improve upon the practices already in place. Manufacturers should use the 4.0 framework to interpret their current state and identify what changes are needed to move to the future state (just like any traditional CIP project), but do not let the daunting task of implementation scare top management away.
Data:
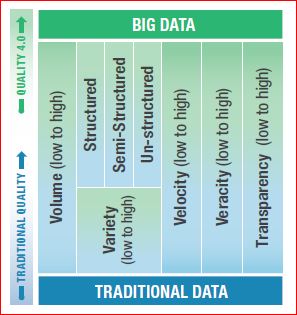
Deming said it best: “In God we trust, all others must bring data.” Data has been driving quality decisions, change, and improvement for a very long time and Evidence-based decision making has become less an anomaly and more the standard. Still, industry has a long way to go toward fully integrating the quality culture. As can be seen in the chart, a portion of the more mature companies have mastered traditional data and have begun leveraging big data. However, the struggle is still genuine and not yet a true cohesive culture across all areas internally or across industries.

Data has five critical elements that must be captured from a practical and cultural perspective-
VOLUME: Traditional systems have a large number of transactional records (e.g., corrective and preventive action (CAPA), NCRs, Change Orders, etc.). The volume of data from connected devices is many orders of magnitude more significant and will continue to grow, requiring specialized approaches such as data lakes, and cloud computing
VARIETY: Systems gathers three types of data: structured, unstructured, and semi-structured. Structured data is highly organized (CAPAs, quality events). Unstructured data is un-organized (e.g., semantics data, data from sensors, and connected devices). Semi-structured data is unstructured and has had structure applied to it (e.g., metadata tags).
VELOCITY: This is the rate at which a company gathers data.
VERACITY: This refers to data accuracy. Quality system data is often low fidelity due to fragmented systems and lack of automation.
TRANSPARENCY: The ease of accessing and working with data no matter where it resides or what application created it.
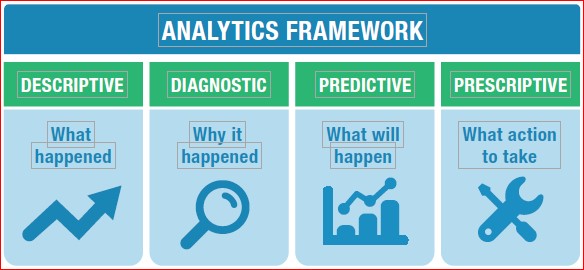
Analytics:
Analytics are the tools that reveal what the terabytes of data can tell us. Unfortunately, quality often stumbles over analytics. 37% of the market identifies weak metrics as a top roadblock to accomplishing quality objectives. Because there is insufficient adoption of real-time metrics by most of the market, we are often acting on the past, not the current situation.
Analytics fall into four categories- Descriptive (number of open quality events), diagnostic metrics (quality process cycle times to identify bottlenecks), Predictive metrics such as trend analysis (application of trend rules to SPC data), and Predictive using Big Data analytics, or Machine Learning (ML)/AI to traditional data or Big Data (predicting failures of a specific machine or process).
Companies attempting to achieve Quality 4.0 should construct their analytics strategy after or concurrently with their data strategy. Powerful analytics applied to low veracity data will only provide low veracity insights.
Connectivity:
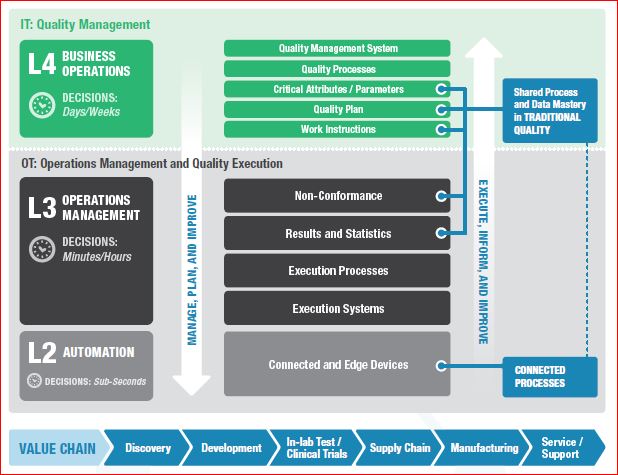
“Connectivity” in the modern industrial age is the cascading multi-direction connection between business information technology (IT) and operational technology (OT), enterprise quality management system (EQMS), enterprise resource planning (ERP), and product lifecycle management (PLM), with OT at the level of technology used in laboratory, manufacturing, and service. Industry 4.0 transforms connectivity through a proliferation of inexpensive connected sensors that provide near real-time feedback from connected people, products and edge devices, and processes.
- Connected people can leverage personal smart devices or intelligent wearable devices that sense workers or their environment. The Connected worker programs usually have goals of increased efficiency and safety.
- Connected products can provide feedback on their performance across their lifecycle.
- Connected edge devices efficiently connect sensed equipment. Edge devices often perform analytics at the device, helping to make predictive/prescriptive decisions (shut this machine down and come for repair), and decide which data to send to central OT systems.
- Connected processes provide feedback from connected people, products, and equipment into processes. This broad element of connectedness allows for the overall reduction of the decision process by providing accessible data and reliable analytics
Collaboration:
Quality management requires collaboration. Quality is inherently cross-functional and global. With the help of digital messaging (email, IM), automated workflows, and online portals, companies execute traditional quality business processes. Much of the market has yet to take advantage of automated workflows and portals, while only 21% have adopted a core EQMS.
Collaboration is a powerful fuel for innovation and quality improvement and has been profoundly transformed and magnified by connectivity, data, and analytics. Leaders should consider how they collaborate and build a secure and reproducible data sharing strategy that meets objectives such as better competency, more streamlined oversight, improved security, and auditability. The approach of collaboration is often part of the culture, and reproducing it can be difficult.
Scalability:
Scalability is the ability to support data volume, users, devices, and analytics on a global scale. Without a global scale, traditional quality and Quality 4.0 are not nearly as effective, and a company is unable to harmonize processes, best practices, competencies, and lessons learned corporate-wide. Thirty-seven percent of companies struggle with fragmented data sources and systems as a top challenge in achieving quality objectives. Scalability is critical to Quality 4.0
Management Systems:
The EQMS is the Center of all quality management connectivity and provides a scalable solution to automate workflows, connect quality processes, improve data veracity, provide centralized analytics, ensure compliance, and foster collaboration within a universal app. It is a hub because quality touches every part of the value chain and how it’s managed. Over the last 50 years, business has slowly realized that quality was not the bad guy but was, in fact, the helping hand to allowing us to have the capacity to remove much of the hidden factory.
There has been some progress on EQMS adoption, but many companies are still critically lagging. Even those that adopt EQMS have not utilized it in an integrated fashion. Only 21% of the market has adopted EQMS, and of those, 41% have adopted a standalone unintegrated approach, leading to fragmentation. Fragmented core processes and resistance-to-change create the current situation. While CAPA/non-conformance is globally harmonized at 25%, 36% of manufacturers have not harmonized any processes, and the median harmonization rate of a single process is an abysmal 8%
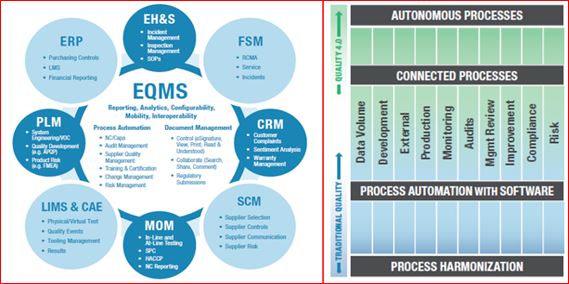
Compliance:
Compliance would include conforming to regulatory, industry, customer, and internal requirements. Life science manufacturers/ have a particularly heavy compliance burden, but many other sectors are even more burdened by compliance with regulations. Compliance is essential to quality teams across all industries since quality often takes a lead role in ensuring that processes, products, and services not only conform with requirements but are safe for the public. Manufacturers already leverage technology in every way possible to help reduce the cost and effort to comply (work smarter, not harder). Initial attempts at implementation of compliance technology required considerable custom code to address requirements. While helping to achieve compliance, custom code was difficult to upgrade and revalidate. This result was often known as “version lock,” where companies chose to delay upgrades by many years to avoid the cost and effort of migrating and revalidating code and data.

Quality 4.0 provides further opportunities to automate compliance. Active real-time collaboration offers a mechanism to share successful and failed approaches to compliance across groups, sites, and regions. Analytics can be used to implement internal alerts and preventative measures for organizations to automatically address potential compliance breaches or act to prevent the violations. Integrated IT/OT data models and/or collaboration technologies like blockchain can provide a data-driven approach that automates auditability.
Culture:
Leaders should feel the drive to develop a Culture of Quality since Quality often owns the ultimate responsibility for process execution with insufficient cross-functional participation and ownership from other functions. A company that has “a Culture of Quality” exhibits four key elements: process participation, responsibility, credibility, and empowerment. Companies need to set goals for cross-functional process participation, cross-functional responsibility for Quality, credibility for Quality and its work across functions, as well as cross-functional empowerment. In traditional settings, achieving all of this concurrently can be quite tricky, in part due to regulatory burden, weak metrics and metric visibility, fragmented data systems and sources, (not to mention fragmented processes). Quality often presents itself to employees as a giant maze of regulation and rules; more like the “quality police” than CIP. Only 13% of cross-functional teams clearly understand how Quality contributes to strategic success. Quality 4.0 can make a culture of Quality much more attainable through better connectivity, visibility, insights, and collaboration. Connected data, processes, analytics, apps, etc., will help to improve the Culture of Quality through shared/connected information, insights, and collaboration. Quality 4.0 makes quality processes and outcomes more visible, connected, and relevant. Community is the primary component of Culture.

Conclusion
Manufacturers looking to improve quality should assess where they stand on each of the key Axes of Quality 4.0 and prioritize investments for the long term. Given the current state of Quality in the market, it is probable that many companies will find themselves required to make investments first in traditional quality before they can fully become part of Quality 4.0. If the foundation (Quality 3.0) is not yet fully developed, any company would be foolish to build on shifting soil.
There are clear interrelationships among the axes, and any company that is willing to add new capabilities to individual axes enables new applications on others. Quality 4.0 makes critical new technologies affordable and accessible, and the story of Quality 4.0 is really about the application of these technologies to solve long-standing quality challenges and to reoptimize to provide novel solutions. Quality 4.0 is real, gaining momentum, and inevitable. Quality leaders should prioritize Quality 4.0 plans. Those that stay on the sidelines are at a high risk of being left behind- but follow the right game plan and don’t fall for buzzword packaging that promises “a new kind of quality.” You will have to invest but invest in yourself, and with those who genuinely know Quality. If you do not, those executives eager for a quick financial return will ok the “solve all your problems” fix solicitors try to sell you. When it fails, the natural resistance to organizational change will kick in double and it will be even harder to convince anybody that the change is worth re-initiating. “Do It Right The First Time” (-John Wooden)
The paper I got the most comprehensive information from was QUALITY 4.0 IMPACT AND STRATEGY HANDBOOK (from LNS Research), which detailed 11 axes of quality. Still, I only addressed the 8 I felt were pertinent to most quality professionals, but the link to the white paper can be found in the bibliography. I would recommend the Handbook if you need a more detailed evaluation of Quality 4.0 and future requirements (Dan Jacob, 2017).



Bibliography
ASQ. (n.d.). The History of Quality. Retrieved from https://asq.org/quality-resources/history-of-quality
Dan Jacob. (2017). Quality 4.0 Impact and Strategy Handook. Retrieved from https://www.sas.com/content/dam/SAS/en_us/doc/whitepaper2/quality-4-0-impact-strategy-109087.pdf
George Mason University. (2014). Mercatus Center. Retrieved from https://RegData.org