
The Process Audit Framework

REFLECTION: FOR STUDENTS: Be mindful in your scholarly efforts. Mindfulness will reflect well both as auditee and an auditor
FOR ACADEMICS: Teach the art of communication, for clear communication is fast becoming a lost tool, but in the future good communication skills will be golden
FOR PROFESSIONALS/PRACTITIONERS: Anyone who has endured an audit knows what a burden it can seem to be. My advice would be to take every audit as an opportunity to improve your process. Do not try to slip by simply by knowing when to keep quiet, but be open and honest with the auditors and your process, improve, and then repeat. Eventually your process will withstand all but the most biased audit process.
If you are an auditor, do not go into the audit searching for failure. Those are the auditors who find themselves asking “why is the factory hiding everything from me?”. Well, it may be because they know you see everything possible as a failure because you have already failed them and they know it. Be more objective and your audit will go much easier.
Process Audit
The general definition of a Process is – any series of interrelated steps that, when performed in sequence, turns inputs into outputs. A process usually adds value with its output, but when evaluated overall, a process may not add value based upon non-value-added costs in the process. Because processes requirements are not always adhered to, and because processes are not always designed to produce the optimum output with maximum efficiency, frequent process audits should be performed to confirm that the process is being adhered to per the SOP (Standard Operating Procedure) and that opportunities for improvement are highlighted so that process improvement can begin.
An excellent way to approach a process audit is to look at the process that you are auditing as a set of components: inputs, process performance, and outputs. Looking at the process from the 6M’s you can develop questions around each of the M’s
- Man
- Do operators demonstrate competence in the operation?
- Are all operators trained?
- Machine
- Is all equipment calibrated?
- All equipment validated as required?
- Does equipment meet TPM schedule?
- Method
- Do the methods employed for production match SOPs/WIs?
- Are all SOPs/WIs to current Rev?
- Are any procedures contributing to bottlenecks or potential NCs?
- Material
- Are all Materials correct and traceable?
- Any issues with defective materials?
- Measurement
- Is there an active Data Collection Plan?
- Any discrepancies with data?
- Mother Nature
- Environmental control in-spec?
- Any Safety Hazards observed?
Process Performance and Monitoring
- Performance
- Process controlled?
- Are outputs as expected per process?
- Monitoring
- Data Recorded as required?
- Data Trending properly?
- Effective monitoring in place?
Process Outputs
- Products
- Meets spec?
- Yields as expected?
- Records
- All required available?
- Complete?
- Correct?
- GDP followed per company requirements?
- Signals
- Alerts, alarms, status signals sent as required?
- Consequences (risks)
- Unexpected or expected consequences documented and evaluated?
- Contingencies in place?

(Lance B. Coleman, 2015)
High level Process Audit Framework-
- Audit Preparation
- Audit Execution
- Audit Report
- Retention of Audit Records
- Audit Completion
- CAPA follow up (as required)
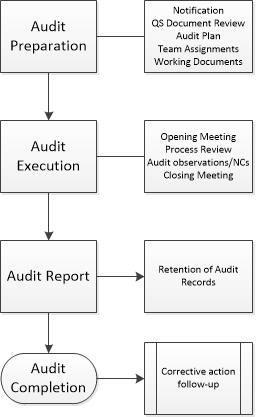
Audit Preparation
Unless regulatory requirements mandate otherwise, the Lead Auditor should notify the auditee in advance of a coming audit. The Lead auditor should also evaluate the quality system to determine if the implemented controls identified in the process meet regulatory requirements. The most effective way to accomplish this review is to assess the quality manual and top-level SOPs. If it is clear that the system audited does not meet regulatory requirements, the auditee should be informed the audit will be suspended until the required corrective actions are complete. The Lead auditor should develop an audit plan to be submitted to the auditee for approval before the audit (where allowed per regulations). The Plan should define the objectives and scope of the audit, identify the auditee’s management team having direct responsibility for the audit scope and purpose. Identify the applicable standards to be used, the Process(es), the lead auditor and audit team members, specify language of the audit, time frame and locations, all organizational units and areas to be audited, schedule meetings with auditee management, and identify who receives the final audit report (and when) (Crawford, 2017).
Audit Execution
Audit Execution begins with the opening meeting, including senior management. The purpose of this meeting is-
- Introduce the audit team
- Fully clarify the audit scope, objectives, plan, and schedule
- Review the methods and procedures to be used during the audit
- Confirm that the resources required for the audit are available
- Establish firm paths of communication
(Crawford, 2017)
Process Assessment begins with applicable elements of the process and quality system evaluated. Objective evidence is collected by:
- Interviewing and questioning personnel
- Reviewing records and documents
- Visually observing activities and conditions
An excellent little acronym for auditing is D.O. o R. S, representing doors to remembering types of audit evidence. D.ocuments or documented information are organizational policies, SOPs, WIs, drawings, or any other guidance document. O.bservations represents everything witnessed by the audit team. R.ecords are completed forms maintained, providing a historical record of organizational activity relative to the process. S.tatements include interview responses, explanations of procedures, or overheard conversations (Lance B. Coleman, 2015).
If possible, evidence collected via personal interviews should be confirmed by other methods. Any Documents or copies of records collected or any photos taken during the audit should be noted by the auditor and acknowledged by the auditee. Upon evaluation of the objective evidence collected, auditors must document their quality audit observations against the process.
Nonconformities (non-fulfillment of requirements per the process or regulations) and Observations that could become nonconformities should be reviewed as they are observed. The collected objective evidence must support any Nonconformity and identify what requirement has been violated.
A closing meeting should be held with the management and process owner, clearly defining all quality observations, nonconforming findings, and categorizing each finding by severity from Opportunity For Improvement, Minor NC, to Major NC. Positive comments should be noted, as well.
The Supervisor, Management, and all invited parties will, at this point, have an opportunity for a final discussion and then be presented with the list of observations, NCs, and feedback(Crawford, 2017).
Audit Report
The Audit Report is formal communication. The lead auditor will finalize the report based upon the overall objective evidence and the final discussion. Any NCs in the report should be very specific so the party being audited can more readily address the issue, and should include-
- Requirements of the internal or external standard
- Severity
- The required date for submission of a corrective action plan
Keep clear lines of communication open(Crawford, 2017).
Audit Completion
The audit should not be considered complete and closed until any associated corrective actions have been completed and communicated to the auditor. Auditing documents should be retained or destroyed by agreement between auditee and auditor. In the case of internal audits, process audits are much less formal, but this is a better opportunity for process improvement, so more rigorous process audits should be used internally (Crawford, 2017).
Conclusion
A well planned and executed audit should always be a friendly encounter. A process is not just an object to potentially find fault with but is usually the critical point of possible improvement in an organization. When auditing, be helpful to the auditee in pointing out Opportunities for Improvement -OFIs- (fresh eyes usually see most clear), and when being audited, do not be fearful of findings. When the finding is justified, accept it and act upon the finding, correct the issue, and as often as possible act upon the OFIs as well.


Bibliography
Crawford, H. (2017). The Biomedical Quality Auditor Handbook. Milwaukee, WI: Quality Press.
Kubiak, T. a. (2017). The Certified Six Sigma Black Belt Handbook Third Edition. Milwaukee: ASQ Quality Press.
Lance B. Coleman, S. (2015). Advanced Quality Auditing. Milwaukee, WI: ASQ Quality Press.